Navigating MOET and IVF
Boost Your Breeding Success: Navigating MOET and IVF Choices for Optimal Cattle Reproduction
Seedstock producers can today harness IVF (In vitro fertilisation), FSH-stimulated IVF and MOET (Multiple Ovulation Embryo Transfer) to fast-track genetic improvement by increasing the number of progeny from superior females. Understanding the full spectrum of these options including the impact of follicle stimulating hormone (FSH) is crucial for informed decision-making that aligns with both economic and genetic goals.
Recent reports by the International Embryo Technology Society (IETS) and the American Embryo Transfer Association (AETA) underscore the pivotal role of in vitro techniques in advancing cattle genetics.1


For producers, the choice of reproductive strategy goes beyond a technical decision to envisioning the herd's future. Ultimately, whichever strategy a producer chooses, the goal is to produce a high genetic merit calf capable of performing to its full potential.2 Both MOET and IVF excel in optimising offspring from superior dams and sires. However, each method offers distinct benefits, necessitating a clear understanding in order to choose the most advantageous approach.
The pivotal distinction lies in where embryo development occurs: MOET matures and fertilises embryos inside the donor cow, while IVF accomplishes this in a lab setting. Good quality oocytes selected at the right time of development play a crucial role in successful outcomes.3 The central hurdle in assisted reproduction is consistently producing high-quality oocytes that can develop into healthy, implantable embryos.4
Enhancing Embryo Yield: The Role of FSH
Using Follicle Stimulating Hormone in IVF protocols elevates more than just oocyte counts – it enriches oocyte quality, fortifying them for successful fertilisation and subsequent development into blastocysts3. This enhancement is due to the critical role of FSH in synchronising follicular growth, thus yielding oocytes at their optimal developmental stage, leading to more uniform and competent embryos.
Although producers often select MOET due to the higher number of viable embryos collected from a single flush and the higher conception rate of implanted embryos, strategic use of FSH in IVF programs may offer similar advantages.5

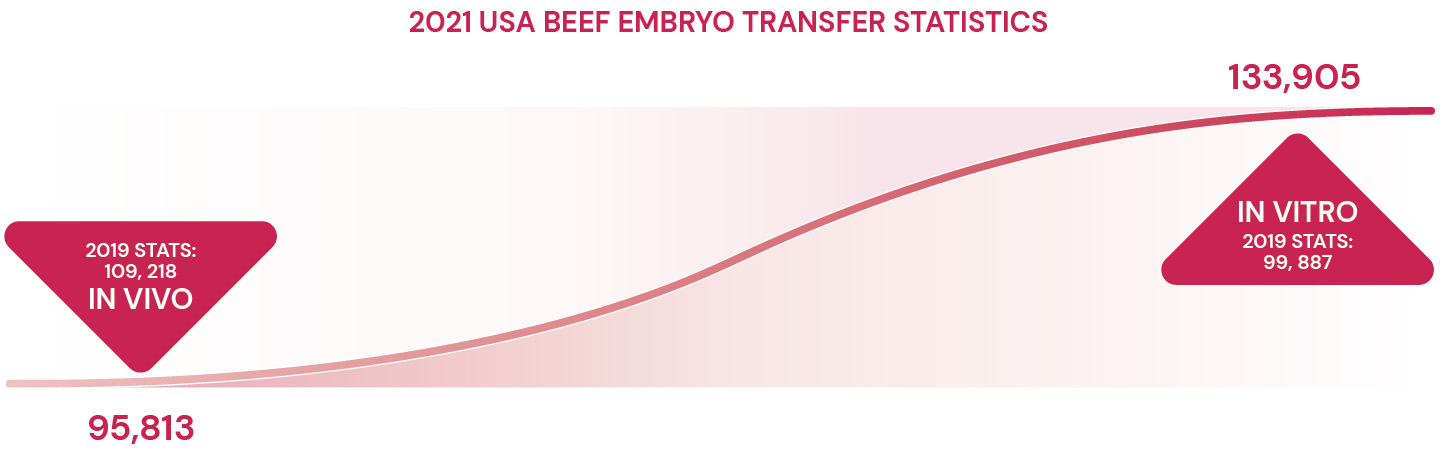
Real-world studies back the benefits of FSH, showing a significant 55% boost in viable embryo production with FSH-enhanced IVF, as opposed to standard protocols.8 AETA’s findings echo this, indicating substantial gains in embryo viability -- 68% in beef cattle and an impressive 105% in dairy cattle, highlighting FSH’s significant role.9 Such evidence firmly establishes FSH as a key factor in modern IVF programs, offering benefits that rival traditional MOET outcomes.
The success of FSH in IVF also varies with cattle breeds, underlying the need for breed-specific strategies when choosing reproductive technologies. For instance, Bos indicus breeds excel in IVF settings, yielding 2 to 3 times more oocytes per IVF collection than Bos taurus breeds.10 This higher oocyte production makes IVF particularly cost-effective for Bos indicus, pointing to the necessity of a customised approach in reproductive technique selection to align with specific breed attributes.
Strategic Selection
Selecting the right reproductive technology for a herd or individual animals requires a balanced consideration of the benefits and limitations inherent in IVF, FSH-stimulated IVF and MOET. The increased adoption of IVF in the Americas is attributed to its ability to generate viable embryos in a shorter time frame than MOET. This is largely because IVF permits oocyte collection at frequent intervals – every 7 to 14 days – unlike the extended 30 to 60-day cycles necessitated by MOET, which requires hormonal treatments for superovulation and in-cow embryo production.1,2
IVF’s direct oocyte retrieval from the ovaries streamlines the process, circumventing the need for donor cycle synchronisation and in vivo maturation, making it advantageous in situations where MOET is not viable, such as with young heifers, non-responsive donors, pregnant cattle or when only post-mortem ovaries are available.2 Furthermore, IVF’s flexibility in semen utilisation – allowing for a single semen sample across multiple donors or varying different bulls’ semen on a single donor’s eggs – maximises the use of elite genetic resources and is compatible with sex-sorting semen techniques. 2,11
Although IVF has these operational flexibilities, MOET still maintains a higher reported conception rate ranging from 8 to 27.8% higher than IVF, which can be a deciding factor for many producers.5,12 It is crucial to acknowledge that all breeding methods have inherent risks, such as the embryo’s developing environment influencing the postnatal phenotype.1,13 For instance, 3% of calves born through IVF have been linked to large offspring syndrome, a condition also seen, though less frequently, with MOET and artificial insemination.13
The necessity for specialised equipment and laboratory expertise for IVF also adds a layer of complexity and cost that are less of a concern with MOET, which relies on the natural biological processes of the cow.

Dr Ced Wise, one of Australia’s most respected reproduction experts, emphasises the effect of freezing embryos on conception rates and highlights the breed variation between Bos taurus and Bos indicus, “When we transfer fresh MOET embryos in Bos taurus, we get about 70 % conception rate. In frozen eggs, that's down to about 60%. So, we lose about 10 % [with the freezing process]. In Bos indicus, we're around 60 % if we transfer those fresh. And with frozen, we're down to 40 %.”14Generally speaking, 20% of useable embryos are unsuitable to freeze.14
According to Ced, Australia’s expansive geography can pose significant practical and economic challenges for IVF given “Oocytes must reach the lab within 22 hours of collection, and the resultant embryos must be implanted on the same day they leave the lab. In addition, more than 20 donors may be needed to make an IVF program commercially viable, whereas only one donor is needed with MOET.”
Regardless of modality, Ced also emphasises the importance of
a. fertility and recipient management which he believes are responsible for the biggest variations in pregnancy rates. Producers who start with the most fertile cows in the mob, accurately administer synchronisation drugs and ensure there is optimal nutrition in the two months pre- and post implantation are more likely to achieve optimal outcomes; and
b. maximising pregnancies given the cost of running empty recipients can represent the biggest cost to a MOET or IVF program.
a. fertility and recipient management which he believes are responsible for the biggest variations in pregnancy rates. Producers who start with the most fertile cows in the mob, accurately administer synchronisation drugs and ensure there is optimal nutrition in the two months pre- and post implantation are more likely to achieve optimal outcomes; and
b. maximising pregnancies given the cost of running empty recipients can represent the biggest cost to a MOET or IVF program.
Lastly, producers must consider the limited number of export protocols currently available for IFV embryos.15
Ultimately, the decision between IVF, FSH-stimulated IVF and MOET demands a judicious approach and one that carefully weighs the producer's goals against each method’s strengths and drawbacks. Professional consultation is advised to ensure alignment with specific goals, considering the logistical realities faced by producers, particularly in regions where access to advanced reproductive facilities is limited.
References:
1. Lafontaine S, Labrecque R, Blondin P, Cue RI, Sirard MA. Comparison of cattle derived from in vitro fertilization, multiple ovulation embryo transfer, and artificial insemination for milk production and fertility traits. J Dairy Sci. 2023;106(6):4380-4396. doi:10.3168/jds.2022-22736
2. Lamb GC, Fontes PLP, Oosthuizen N. IN VITRO FERTILIZATION (IVF) VERSUS MULTIPLE OVULATION EMBRYO TRANSFER (MOET): MAKING THE DECISION TO USE ONE OR BOTH.
3. Demetrio D, Demetrio C, Oliveira M, Reis R, Santos R. From oocyte to calf: practical aspects of bovine in vitro embryo production.
4. Blondin P, Vigneault C. Donor preparation for OPU and IVF.
5. Pontes JHF, Nonato-Junior I, Sanches BV, et al. Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology. 2009;71(4):690-697. doi:10.1016/j.theriogenology.2008.09.031
6. Demetrio D. 2020 STATISTICAL INFORMATION COMMITTEE REPORT (2019 DATA). Published online 2020.
7. Steinhauser C, Looney C, Hasler J, Renaud P. Retrospective analysis of superstimulation with Folltropin®-V in Wagyu versus other beef breeds.
8. Santos RM, Oliveira M, Demetrio CGB, Hasler JH, Fonseca JC, Demetrio DGB. 146 Single injection of follicle-stimulating hormone before ovum pickup in lactating Holstein donors: Oocyte recovery and embryo production. Reprod Fertil Dev. 2021;33(2):181. doi:10.1071/RDv33n2Ab146
9. Demetrio D. 2020 STATISTICAL INFORMATION COMMITTEE REPORT (2019 DATA). Published online 2020.
10. Vizoná RG, da Costa Perez B, Campolina Diniz Peixoto MG, et al. Genetic analysis of in-vitro embryo production traits in Dairy Gir cattle. Theriogenology. 2020;148:149-161. doi:10.1016/j.theriogenology.2020.02.014
11. Ferré LB, Kjelland ME, Strøbech LB, Hyttel P, Mermillod P, Ross PJ. Review: Recent advances in bovine in vitro embryo production: reproductive biotechnology history and methods. Animal. 2020;14(5):991-1004. doi:10.1017/S1751731119002775
12. Siqueira LGB, Torres C a. A, Souza ED, et al. Pregnancy rates and corpus luteum-related factors affecting pregnancy establishment in bovine recipients synchronized for fixed-time embryo transfer. Theriogenology. 2009;72(7):949-958. doi:10.1016/j.theriogenology.2009.06.013
13. Hansen PJ. Review: Some challenges and unrealized opportunities toward widespread use of the in vitro-produced embryo in cattle production. animal. 2023;17:100745. doi:10.1016/j.animal.2023.100745
14. Wise C. Assisted Peproductive Technologies in the Cow: Procedures and Comparisons. Published online 2023.
15. p3_admin. What You Need to Know About Embryo Exports. Trans Ova Genetics. Published February 9, 2018. Accessed November 20, 2023. https://transova.com/2018/02/what-you-need-to-know-about-embryo-exports/


