The Dairy Reproductive Revolution: Navigating the Future with MOET, IVP, and FSH
Worldwide trends show the dairy industry embracing innovative breeding strategies and a notable shift toward in vitro production (IVP).1,2 In fact, four times as many embryos are produced this way compared with traditional in vivo methods such as Multiple Ovulation Embryo Transfer (MOET).3
The US Dairy Sector leads the way, with the International Embryo Technology Society (IETS) recently reporting a 33% surge in their IVP cattle embryos, reflecting a robust adoption of IVP to maximise genetic progress and herd productivity (Figure 1).3 Furthermore, combined IETS and American Embryo Technology Society (AETA) data reports that almost half of North American IVP programs were enhanced using follicle stimulating hormone (FSH), and almost all European countries exclusively use FSH (Figure 2).3
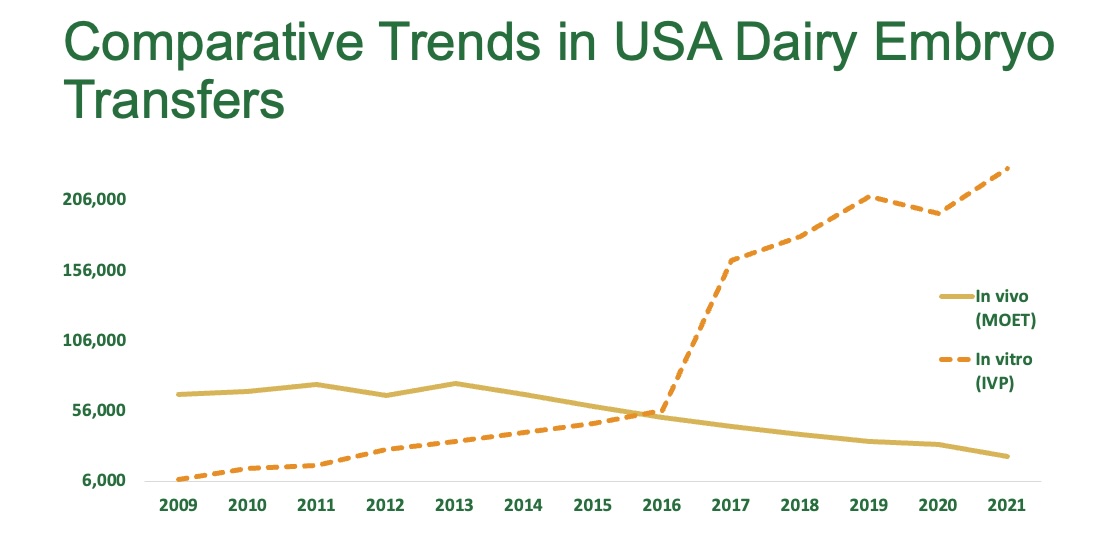
Figure 1: Comparative Trends in USA Dairy Embryo Transfers - adaptation of 2021 IETS data.3
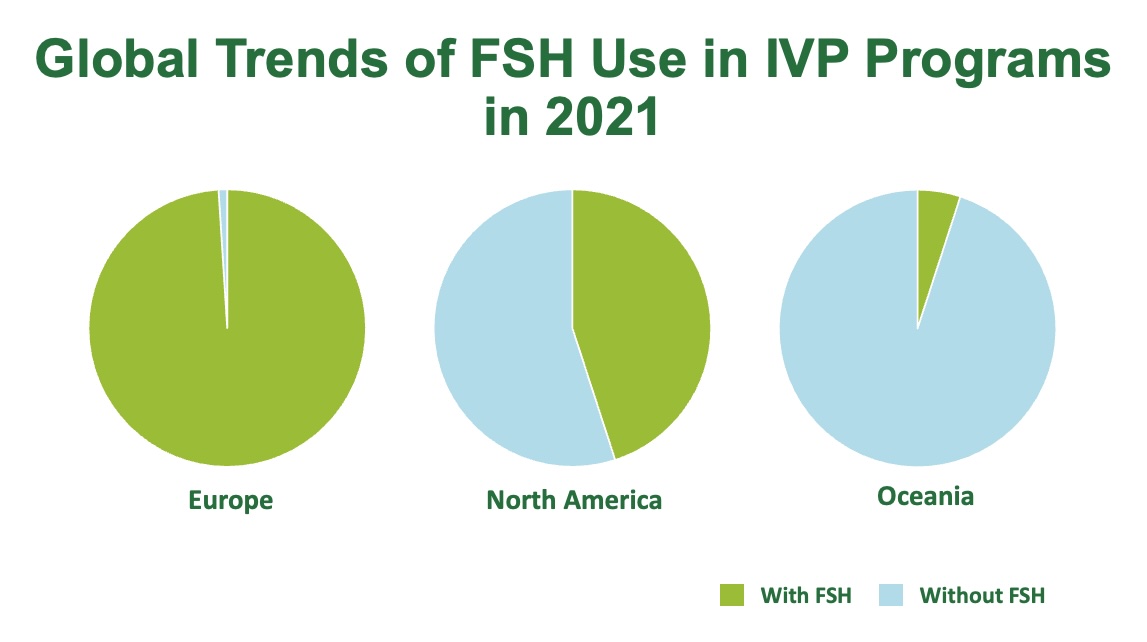
Figure 2: Global Trends of FSH Use in IVP Programs.1,3
Keeping your eyes on the prize
Embryo transfer methods utilising traditional MOET or in vitro production (IVP) – also known as Trans-Vaginal Recovery (TVR) – both excel in optimising offspring from superior dams and sires.4 However, there are some crucial distinctions with each strategy (Figure 3), and it’s essential to make sure you are comparing apples with apples when selecting the best option for your herd, as terminologies between providers differ.

Figure 3: Comparison between MOET and IVP techniques
Producers must keep the purpose of a breeding strategy – producing a high genetic merit calf capable of performing to its full potential – top of mind.5
Both MOET and IVP have the potential to deliver this goal, but there is another often overlooked factor for reproductive success: oocyte quality.6 Successful reproductive programs require high-quality oocytes, selected at the right time of development, that can develop into healthy, implantable embryos.6,7
While MOET programs enable fertilisation and maturation of the oocyte to occur within the natural environment of the cow, IVP collects oocytes, which go through maturation, fertilisation, and more maturation processes in the laboratory before they are ready for freezing or transfer.4
Does it matter where the process takes place? Potentially. IVP commercial success has made great gains over the last decade. However, the literature from the last 25 years indicates that embryos generated in the lab still differ from those developed in MOET, with only 27% of cattle receiving IVP embryos producing a live calf.6
The FSH Effect: Elevating Embryo Yield
Dairy cows typically experience two to three follicular waves during each cycle, and oocytes will be maturing and developing at various stages simultaneously. FSH regulates a crucial part of oocyte development by synchronising follicular growth, ensuring oocytes reach their optimal development stage, leading to more uniform and competent embryos.6
Incorporating FSH into IVP programs improves oocyte yield and quality, resulting in more higher-grade embryos per pick-up in donors.8,9 In fact, using FSH improves oocyte quality to the extent that high pregnancy rates, similar to in vivo methods, can be achieved with this technique.6
The Future for Oceania
Early data indicates Oceania will follow in the footsteps of the Americas by leaning toward IVP reproductive strategies.3 Operational flexibilities such as frequent collection intervals and little to no donor preparation streamline breeding programs, appealing to time-poor farmers.10 In addition, wider donor options, such as young heifers, non-responsive donors, and pregnant cattle, may also appeal.
IVP also provides flexibility in semen utilisation, maximising the use of elite genetic resources and is compatible with sex-sorting semen techniques.5 Such flexibility in semen use has allowed producers to leverage the beef-on-dairy trend, which produces offspring that benefit from the superior meat yield traits of beef cattle whilst better mitigating the negative impact of beef size on dairy cows’ future reproductive performance.11 The convergence of IVP and beef-on-dairy breeding presents unique opportunities for efficient semen use.12 A single semen sample from a premium beef sire may be used across multiple dairy donors, optimising the genetic impact of top-tier beef sires across the dairy herd.12 Equally, different bulls’ semen may be used on a single donor in an IVP setting, allowing for a diverse range of genetic combinations and the potential to tailor offspring based on specific production goals.12
Despite these flexibilities, MOET maintains superior pregnancy rates compared with IVP, which can be a deciding factor for many producers.13 Indeed, European countries select MOET programs three times more often than they do IVP, likely for this very reason.3
Producers in Oceania can benefit from the experience of the global industry and might consider adopting an integrated approach by incorporating the robust capabilities of FSH into IVP programs. Such a strategy elevates the effectiveness of IVP to that of MOET but aligns with the operational needs and time constraints of the modern dairy farm.
Ultimately, reproductive strategies must be tailored toward the individual producer’s goals, and professional consultation is advised to ensure a comprehensive and comparative evaluation of available options.
References
1. Demetrio D. 2022 STATISTICAL INFORMATION COMMITTEE REPORT (2021 DATA). Published online 2022.
2. Sanches BV, Zangirolamo AF, Seneda MM. Intensive use of IVF by large-scale dairy programs. Anim Reprod. 2019;16(3):394-401. doi:10.21451/1984-3143-AR2019-0058
3. Viana JH. 2021 Statistics of embryo production and transfer in domestic farm animals. N Am. Published online 2022.
4. Mueller ML, Van Eenennaam AL. Synergistic power of genomic selection, assisted reproductive technologies, and gene editing to drive genetic improvement of cattle. CABI Agric Biosci. 2022;3(1):13. doi:10.1186/s43170-022-00080-z
5. Lamb GC, Fontes PLP, Oosthuizen N. IN VITRO FERTILIZATION (IVF) VERSUS MULTIPLE OVULATION EMBRYO TRANSFER (MOET): MAKING THE DECISION TO USE ONE OR BOTH.
6. Demetrio D, Demetrio C, Oliveira M, Reis R, Santos R. From oocyte to calf: practical aspects of bovine in vitro embryo production.
7. Blondin P, Vigneault C. Donor preparation for OPU and IVF.
8. Santos RM, Oliveira M, Demetrio CGB, Hasler JH, Fonseca JC, Demetrio DGB. 146 Single injection of follicle-stimulating hormone before ovum pickup in lactating Holstein donors: Oocyte recovery and embryo production. Reprod Fertil Dev. 2021;33(2):181. doi:10.1071/RDv33n2Ab146
9. Sarkar D, Teja A, Debbarma K, et al. Advanced assisted reproductive technology in cattle: OPU-IVF review. INDIAN J Anim Health. 2021;60(2). doi:10.36062/ijah.2021.06321
10. Lafontaine S, Labrecque R, Blondin P, Cue RI, Sirard MA. Comparison of cattle derived from in vitro fertilization, multiple ovulation embryo transfer, and artificial insemination for milk production and fertility traits. J Dairy Sci. 2023;106(6):4380-4396. doi:10.3168/jds.2022-22736
11. Berry DP. Invited review: Beef-on-dairy—The generation of crossbred beef × dairy cattle. J Dairy Sci. 2021;104(4):3789-3819. doi:10.3168/jds.2020-19519
12. Ahmed RH, Schmidtmann C, Mugambe J, Thaller G. Effects of the Breeding Strategy Beef-on-Dairy at Animal, Farm and Sector Levels. Animals. 2023;13(13):2182. doi:10.3390/ani13132182
13. Siqueira LGB, Torres C a. A, Souza ED, et al. Pregnancy rates and corpus luteum-related factors affecting pregnancy establishment in bovine recipients synchronized for fixed-time embryo transfer. Theriogenology. 2009;72(7):949-958. doi:10.1016/j.theriogenology.2009.06.013

